MacrolactoneDB is a central repository of macrolactones and their bioactivities. It enables machine learning to inform synthetic biology and medicinal chemistry efforts for designing new/better drugs. Our new paper in Nature Scientific Reports is the result of a collaboration with Phyo Phyo Kyaw Zin and Sean Ekins (Collaborations Pharmaceuticals).
Author Archives: gjwillia
SIME: synthetic insight-based macrolide enumerator to generate the V1B library of 1 billion macrolides
Excited to announce our latest publication and collaboration with the Fourches group in the NC State Chemistry, “SIME: synthetic insight-based macrolide enumerator to generate the V1B library of 1 billion macrolides”. This advance allows in silico libraries of macrolides to be constructed based on the knowledge of polyketide biosynthesis. It’s a first step towards the generation of new bioactive macrolides by combining synthetic biology and computational chemistry.
Genetically Encoded Biosensor for Detection of Polyketide Synthase Extender Units in Escherichia coli
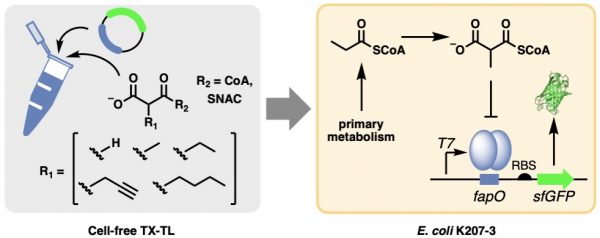 Our latest paper in ACS Synthetic Biology describes the first genetically-encoded biosensor for detection of polyketide synthase extender units in cells. Because the biosensor is genetically encoded, the cell makes all of the necessary components and the entire system can be subjected to directed evolution. A biosensor for detection of various acyl-CoA’s will help synthetic biology approaches for sustainable production of natural products in microbes.
Our latest paper in ACS Synthetic Biology describes the first genetically-encoded biosensor for detection of polyketide synthase extender units in cells. Because the biosensor is genetically encoded, the cell makes all of the necessary components and the entire system can be subjected to directed evolution. A biosensor for detection of various acyl-CoA’s will help synthetic biology approaches for sustainable production of natural products in microbes.
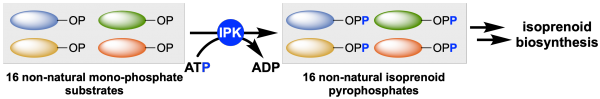
Substrate promiscuity of isopentenyl phosphate kinase
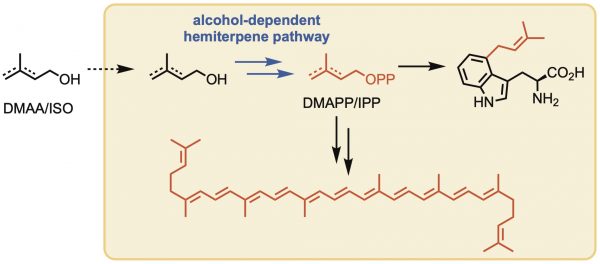
Isoprenoids are a large class of natural products with wide‐ranging applications. Synthetic biology approaches to the manufacture of isoprenoids and their new‐to‐nature derivatives are limited due to the provision in Nature of just two hemiterpene building blocks for isoprenoid biosynthesis. To address this limitation, artificial chemo‐enzymatic pathways such as the alcohol‐dependent hemiterpene pathway (ADH) serve to leverage consecutive kinases to convert exogenous alcohols to pyrophosphates that could be coupled to downstream isoprenoid biosynthesis. To be successful, each kinase in this pathway should be permissive of a broad range of substrates. For the first time, we have probed the promiscuity of the second enzyme in the ADH pathway, isopentenyl phosphate kinase from Thermoplasma acidophilum, towards a broad range of acceptor monophosphates. Subsequently, we evaluate the suitability of this enzyme to provide non‐natural pyrophosphates and provide a critical first step in characterizing the rate limiting steps in the artificial ADH pathway.
See the publication at ChemBioChem!
New paper in the J Am Chem Soc!
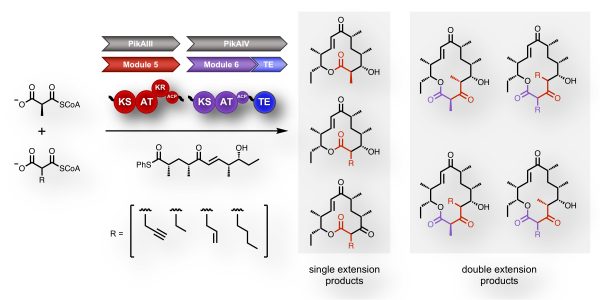
In a new paper in JACS by recent graduate Edward Kalkreuter (now a postdoc at Scripps, Florida), the synthetic scope of a polyketide synthase was expanded by engineering the incorporation of consecutive extender unit substrates. Unexpected downstream bottlenecks were also revealed, identifying new targets for enzyme engineering.
An artificial pathway for isoprenoid biosynthesis
Nature makes isoprenoids via two lengthy routes that are difficult to engineer. In a new publication in ACS Synthetic Biology, Sean Lund and Rachael Hall describe using synthetic biology to develop a third route that is just 2-enzymes long, easy to engineer, and is highly versatile.