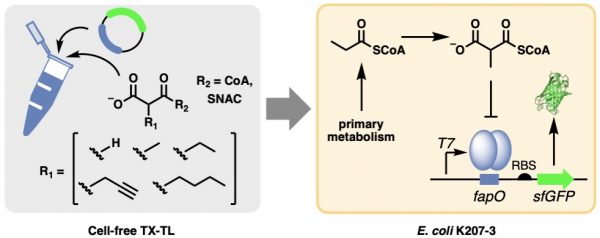
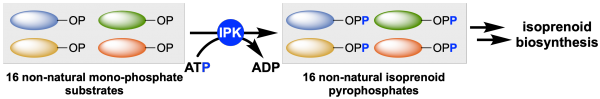
“Polyketide synthases are multi-domain enzymes that catalyze the construction of many bioactive natural products. Now, some of the inefficiencies and limitations of these systems have been solved by designing an artificial pathway for carbon–carbon bond formation via iterative rounds of non-decarboxylative thio-Claisen reactions.”
Grateful for the opportunity along with Anuran Kumar Gayen and Lindsay Nichols to discuss Ramon Gonzalez’s excellent Nature Catalysis research article on artificial polyketide biosynthesis.
See Ramon’s article at https://lnkd.in/dn-sEqC and our News & Views piece at https://rdcu.be/b5F53.